Sublingual Semaglutide

Compounded Sublingual Semaglutide is an oral GLP-1 that is held under your tongue for 5 minutes or more daily, and absorbed into the bloodstream rather than an injectable form that requires injection of a needle to take. We make this in a patent-pending base that helps absorb the larger semaglutide molecule
As of April 22, 2025, the compounded injectable semaglutide will no longer be available due to the FDA’s declaration of drug shortages being removed. Rather than go without therapy, our sublingual is a good alternative, although not quite as strong as the injectable. We recommend a cleaner diet and the addition of moderate exercise to all patients whether they were on injectable GLP-1 Rx or sublingual GLP-1 Rx, to help with get you good results.
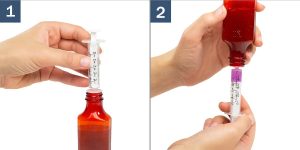 The medication is dispensed in a 30ml bottle with a calibrated 1ml syringe. It should be stored at room temperature and shaken before use. If you are taking Sublingual Semaglutide for the first time, you will be instructed to begin with a titration as directed by your provider. This means that you will start with a smaller dose and increase gradually as tolerated.
The medication is dispensed in a 30ml bottle with a calibrated 1ml syringe. It should be stored at room temperature and shaken before use. If you are taking Sublingual Semaglutide for the first time, you will be instructed to begin with a titration as directed by your provider. This means that you will start with a smaller dose and increase gradually as tolerated.
Titrating this medication helps to reduce gastrointestinal side effects and nausea. Do not use higher doses than stated on your prescription label. It does not matter what time of day you administer the medication, although you must try to hold it for 5 minutes or more under your tongue (never less than 90 seconds!) and NOT EAT FOR 30 MINUTES afterward. View or download our Patient Dosing Guide Here.
See the patient information sheet below. You should discuss this compounded sublingual with your physician and you can download this e-prescribing guide to take to your visit. Your physician can always visit our prescriber page for more information.