Semaglutide – Prescribers
|
Sublingual Compounded Semaglutide Thank you for taking to time to learn about a compounded Semaglutide anhydrous suspension for sublingual administration available from The Medicine Shoppe Compounding Pharmacy that could be of value for your patients. The Medicine Shoppe in Victor, NY is one of Central New York’s most comprehensive compounding pharmacies. We have been custom compounding since the mid 1990s helping patients in all of NY State. We offer free shipping on all our compounded medications or patients can come directly to our pharmacy. We pride ourselves on our customer service and there is always an experienced pharmacist to answer questions from prescribers and patients. While we are a network provider for a most insurance plans and do routinely bill compounds to insurance plans when possible, Please note that, based on the assessments we have made, this compounded medication will not covered by any insurance plans. The usage of GLP-1 receptor agonists, such as semaglutide, has grown exponentially worldwide. This class includes both injectable and oral dosage forms. Many insurances now will not cover GLP-1 medications for weight loss. Also the oral absorption of GLP-1 receptor antagonists is extremely low. (less than 1% per the labeling for RYBELSUS® tablets). For these reasons, prescribers and their patients may prefer an alternative option such as compounded sublingual formulation of Semaglutide. Through our partnership with CMPD Licensing, LLC, an established and innovative compounding pharmacy that developed a proprietary anhydrous sublingual base, we are able to deliver such a compounded medication. Updated Dosing Recommendations Attached here are UPDATED e-scribe guidelines as well as a dosing discussion document. You can download and save or print either of these. New dosing recommendations reflect the following: All Patients,we suggest taking the 4mg/ml as follows: • 0.5ml for 7 days, then increase to 0.75ml for 7 days. Then increase to 1ml ongoing as tolerated. If you have intolerable side effects, consult your prescriber or one of our pharmacists about a dose reductions. We are committed to finding the proper dosage to provide a meaningful outcome for your patients and believe that taking it slowly and titrating is the right way to establish this. We will continue to evaluate and update dosing as more information becomes available. About the Proprietary Base Compounded Injectable Semaglutide 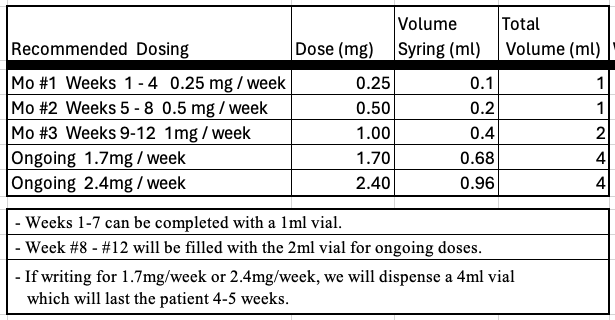 Attached here are e-scribe guidelines as well as a dosing discussion document. You can download and save or print either of these. If your system can’t e-scribe this, please call one of our pharmacists and we will assist you. |
|